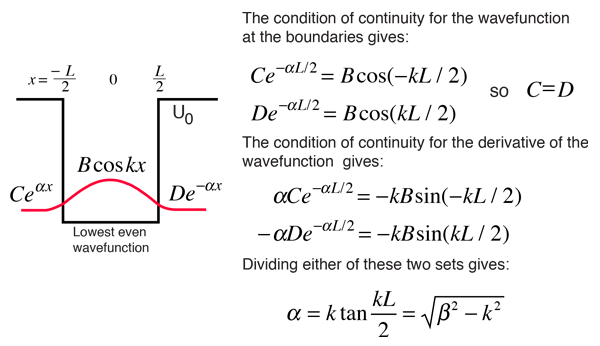
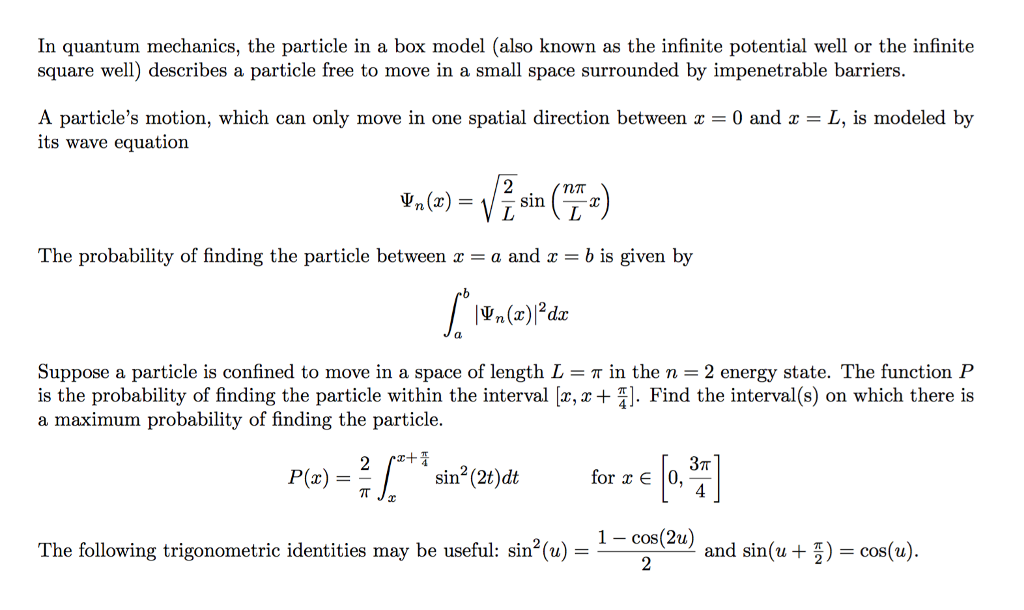
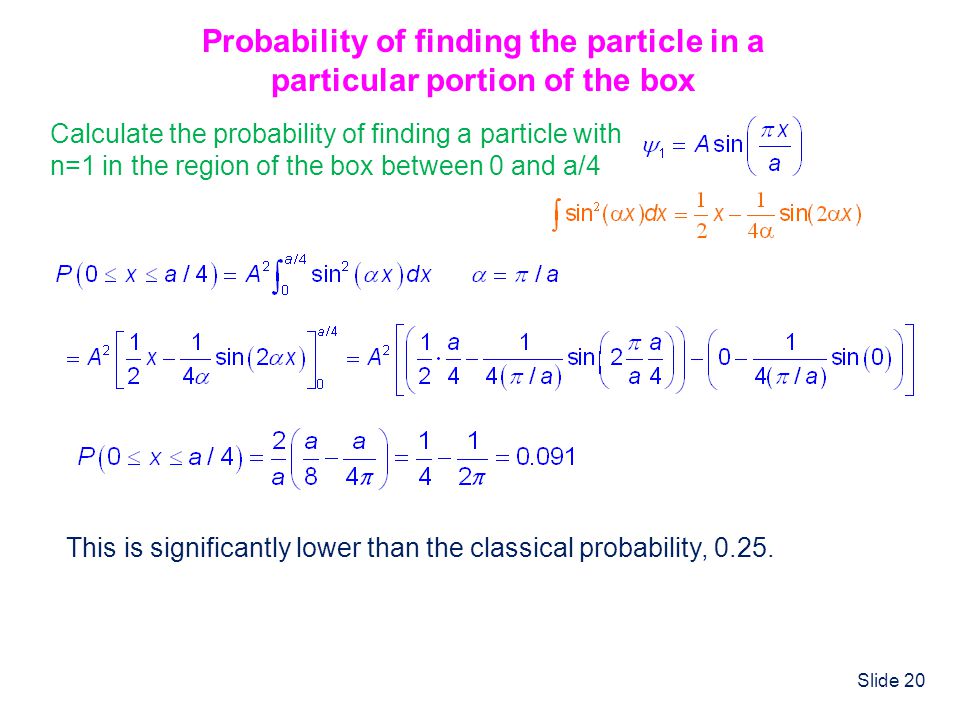
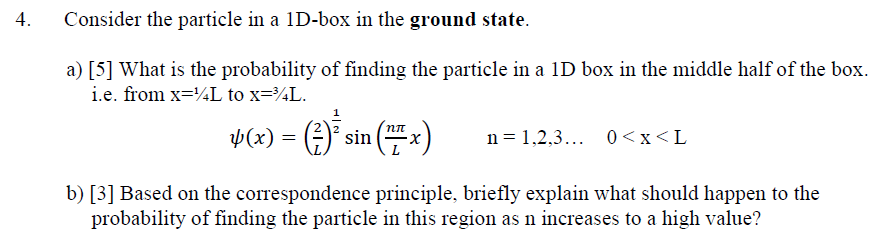
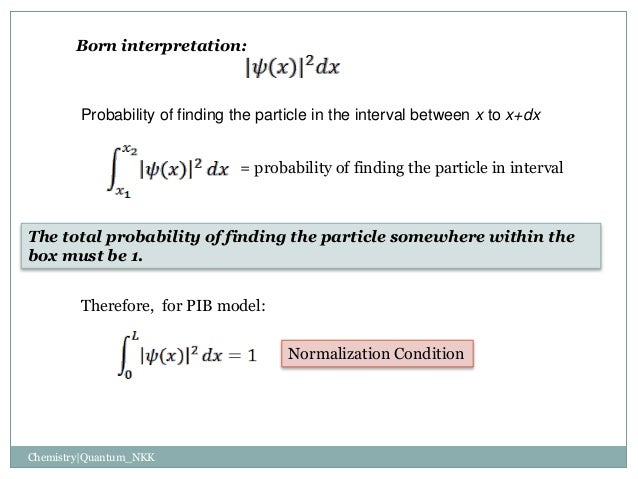
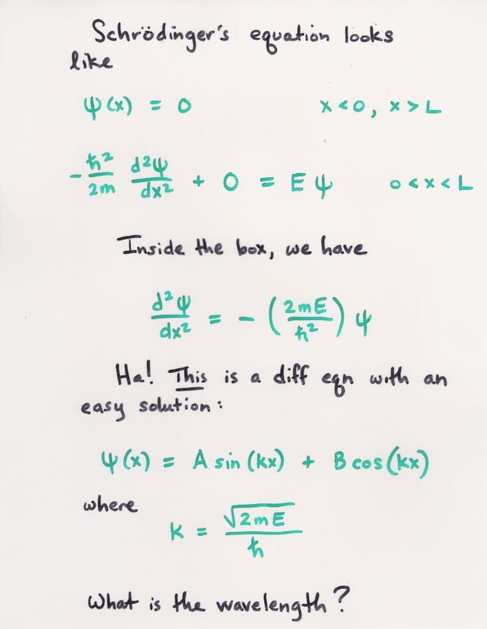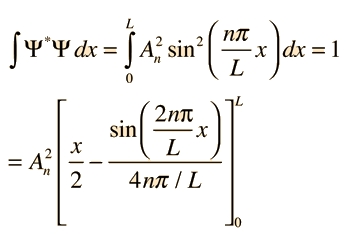Solved] 1) Calculate the probability that a particle in a 1D box of length a will be found between 0.31a and 0.35a for the normalized 1D PIB wavefun... | Course Hero
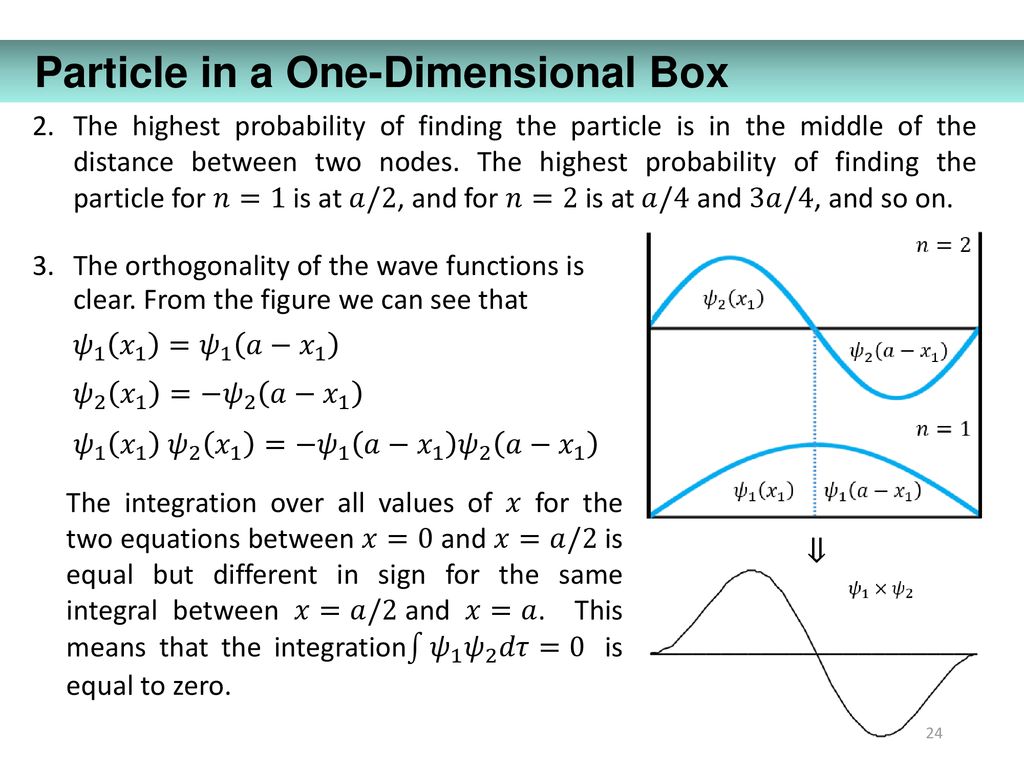
What is the probability of finding a particle in a one-dimensional box in energy level n=4 between x=L/4 and x=L/2? L is the length of the box. - Quora

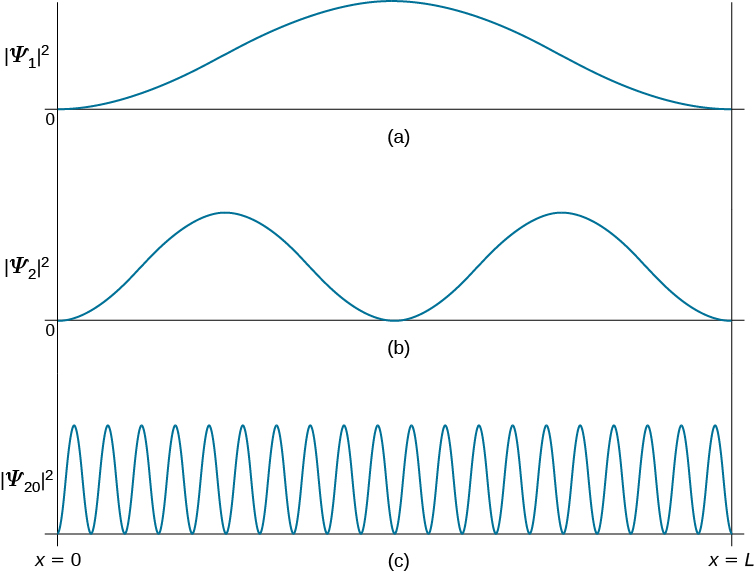
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (26 of 92) Prob. of a Particle 1-D Box n=2 - YouTube

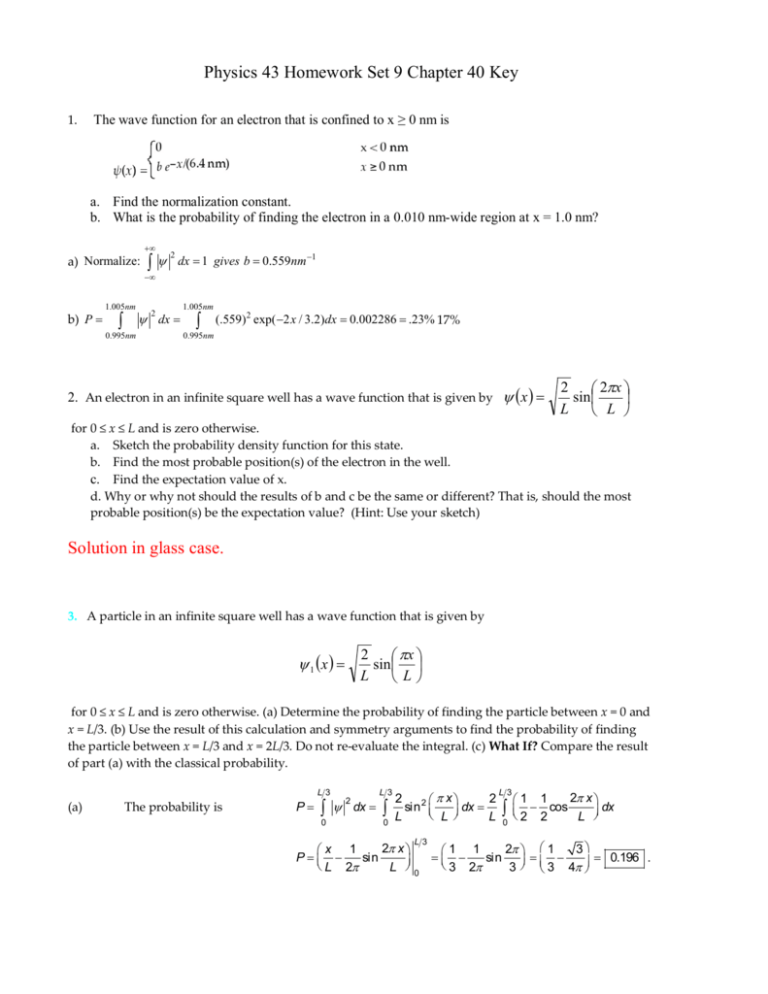
7. What is the probability of finding a particle translating in the central third of a 1 dimensional box if it is in the (a) the ground state (b) the first excited
A particle is confined to a 1D box of unit length.[math] \psi(x) = \sqrt{2} [/math] till x=0.5. Find the probability of the particle having energy E3. - Quora